- Products
- EU pharmaceuticals
The portfolio of EU pharmaceuticals products at axicorp is made up primarily of prescription-only medicines and comprises over 1,600 PZNs (pharmaceutical central numbers). Our broad and demand-focused selection covers a range of indications. These include neurology and multiple sclerosis, diabetes, oncology, rheumatology, HIV, allergy, dermatology, gynaecology and vaccines, to name but a few.
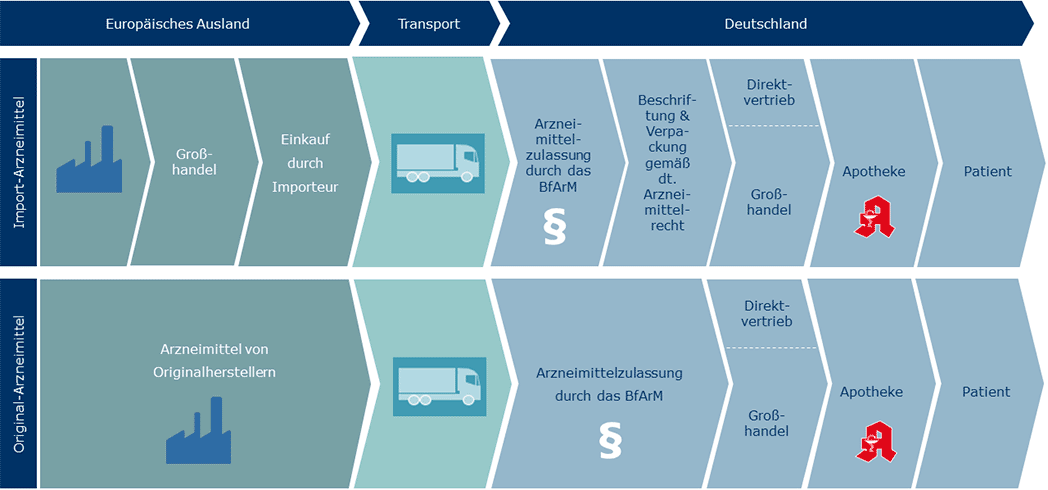
An up-to-date product list can be found in our portfolio summary. At our site in Friedrichsdorf, we repackage the imported original medicines after subjecting them to a quality check and in accordance with German medicines legislation for the German market.
This guarantees high-quality packaging designs appropriate for the original packaging. We also offer many of our medicines in our own, high-quality cardboard boxes.
Nach dem DocCheck-Login gelangen Sie hier zur Sortimentsübersicht.
What are EU pharmaceuticals?
EU pharmaceuticals are original branded medicines from international pharmaceutical companies that are purchased cheaply from other countries in Europe and imported. In terms of effectiveness and quality, they are completely identical to the original preparations and are also subject to all of the laws and standards of German medicines legislation. The lower prices come about through the difference in market prices between other EU countries and Germany. Essentially, there are two forms of import: re-imports and parallel imports.
You will find further information on imported pharmaceuticals at www.die-arzneimittel-importeure.de
What are EU pharmaceuticals?
EU pharmaceuticals are original branded medicines from international pharmaceutical companies that are purchased cheaply from other countries in Europe and imported. In terms of effectiveness and quality, they are completely identical to the original preparations and are also subject to all of the laws and standards of German medicines legislation. The lower prices come about through the difference in market prices between other EU countries and Germany. Essentially, there are two forms of import: re-imports and parallel imports.
You will find further information on imported pharmaceuticals at www.die-arzneimittel-importeure.de
Source: Adapted from http://www.vad-news.de/parallelhandel/funktionsweise/
If there is any suspicion of an adverse drug reaction or an adverse event relating to a medical device, please notify us using our free service number 0800 2940 100 or use the forms below:
If there is any suspicion of an adverse drug reaction or an adverse event relating to a medical device, please notify us using our free service number 0800 2940 100 or use the forms below: